EGFR inhibitors
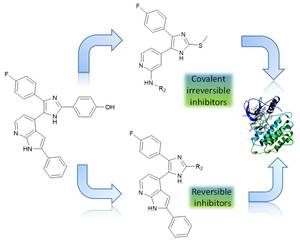
Tyrosine kinase inhibitors (TKIs) offer promising opportunities to fight various types of cancer, especially lung cancer that is among the most deadly cancer types worldwide.[1] First generation EGFR inhibitors (erlotinib, gefitinib) were developed to target non-small-cell lung cancer (NSCLC) of a subgroup of patients, bearing the so-called activating mutation (L858R).[2] After a short period of massive tumor shrinkage, almost all patients develop acquired drug resistance, mainly due to the gatekeeper mutation T790M.[3] Second generation irreversible inhibitors stumbled in clinic due to dose limiting toxicities.[4] Subsequently third generation TKIs (e.g. osimertinib, FDA approved 2015) were developed to overcome T790M resistance through covalent Michael addition to a cysteine side chain (Cys 797) while sparing the wildtype of EGFR.[5] Unfortunately, resistance development to these inhibitors has been discovered.[6] One important mechanism is the point mutation C797S, leading to a massive decrease of inhibitor potency obtaining their majority of efficacy potency through irreversible binding. In our research group we develop a new structural class of EGFR inhibitors (three substituted imidazoles). On the one hand we synthesize reversible inhibitors with improved reversible binding patterns that were able to inhibit all three mutated kinases without the necessity of a covalent tag. On the other hand we developed new irreversible inhibitors with high potency and selectivity in L858R/T790M cell lines, comparable to osimertinib. Because of the strong noncovalent binding properties of our compounds, they barely loose inhibition potency after C797S mutation. Possessing these attractive properties, the molecules may serve as lead compounds for the further development of highly potent L858R/T790M/C797S inhibitors.
Phoenix Wissenschaftspreis 2017 (Kategorie Pharmazeutische/Medizinische Chemie)
Publications:
M. Juchum, M. Günther, S. A. Laufer, Fighting cancer drug resistance: Opportunities and challenges for mutation-specific EGFR inhibitors; Drug Resist Updat. 2015, 20, 12-28.
M. Günther, M. Juchum, G. Kelter, H. Fiebig, S. A. Laufer, Lung Cancer: EGFR Inhibitors with Low Nanomolar Activity against a Therapy-Resistant L858R/T790M/C797S Mutant; Angew. Chem. Int. Ed. 2016, 55, 10890-10894.
[1] R. L. Siegel, K. D. Miller, A. Jemal, Ca-Cancer J. Clin. 2015, 65, 5-29.
[2] A. F. Gazdar, Oncogene 2009, 28, S24-S31.
[3] C.H. Yun, K. E. Mengwasser, A. V. Toms, M. S. Woo, H. Greulich, K. K. Wong, M. Meyerson, M. J. Eck, Proc. Natl. Acad. Sci. USA 2008, 105, 2070-2075.
[4] X. Chen, Q. Zhu, L. Zhu, D. Pei, Y. Liu, Y. Yin, M. Schuler, Y. Shu, Lung Cancer 2013, 81, 155-61.
[5] S. L. Greig, Drugs 2016, 76, 263-273.
[6] K. S. Thress, et al., Nat. Med. 2015, 21, 560-562.
