Biophysics
- Light-harvesting and energy transfer – Photosystem I
- Dynamics of proteins
During the last years we accumulated a wide experience in the spectroscopy of protein complexes of the photosynthetic apparatus. Most of the experiments are performed at the single-molecule level to avoid broadening by ensemble averaging. In addition, the major bunch of the experiments is carried out at cryogenic temperatures (T = 1.4 K). At low temperatures the chromophores are much more photo- and frequency-stable (spectral diffusion) compared to room temperature. In combination with the narrowing of the spectral lines at lower temperatures spectroscopic investigations with high excitation rates become only possible under these conditions.
The origin of protein dynamics and its influence on protein function is one of our special focus. We found that Photosystem I – one of the key-members of the photosynthetic apparatus – has preferential properties for general studies of protein dynamics at the single-molecule level (see Figure 1).
Using Photosystem I, we were able to show that the properties in the inside and the outside of a protein have remarkable influence on its dynamics. An example for the influence of different solutions/matrices on the fluorescence emission of Photosystem I is given in Figure 2 (from a recent publication).
At the moment, a principal hurdle of the expressiveness of optical spectroscopy at low temperatures is the huge amount of cryoprotectants/glassformers that have to be added during sample preparation. This reduces the explanatory power of low temperature spectroscopy. These additives can be omitted if the samples are prepared in a thin water layer. The small the amount of stray light produced by crystalline water interferes the results in this type of samples only slightly.
Based on a comparison of data recorded on protein samples prepared in a thin layer of water and solutions containing additives it becomes possible to estimate the influence of the “traditional” preparation methods (without addition of cryoprotectants) on the spectroscopic results. These experiments are essentially useful to judge about one of the fundamental questions in biophysics since long time: Are the results and the conclusions obtained from low temperatures experiments also valid at ambient temperatures or not?
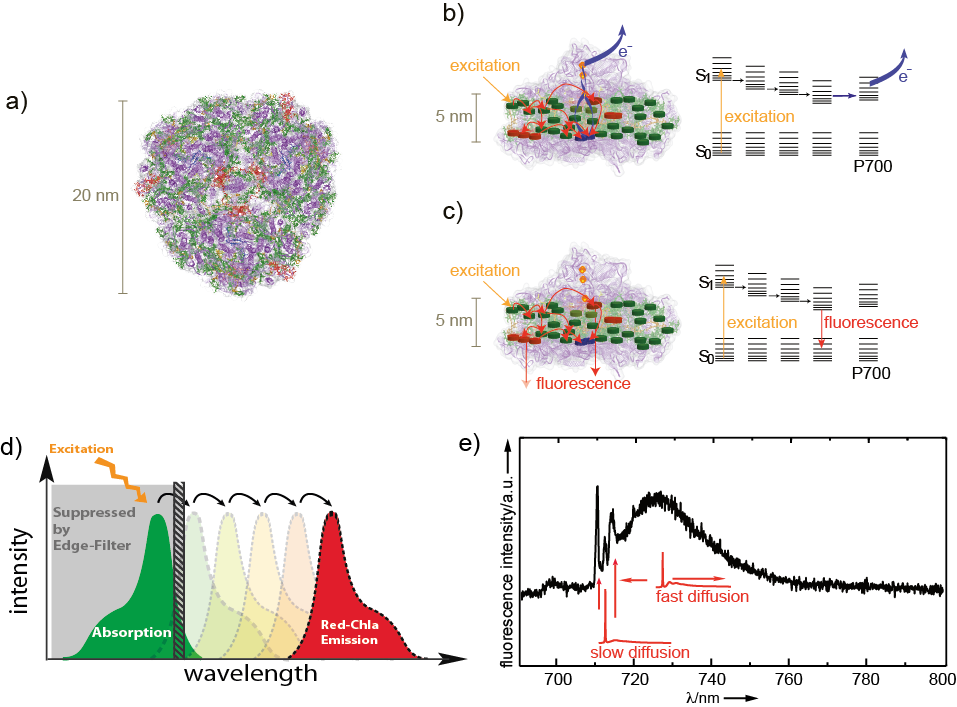
Figure1: Photosystem I a) Top view of trimeric Photosystem I from cyanobacteria. In each mo- nomer about 100 chlorophyll molecules (green) absorb excitation energy and transfer it to a chlorophyll dimer (blue) in the reaction center which absorb at 700 nm (P700). The protein backbone is shown in violet. b) Illustration of excitation-energy transfer pathways at ambient temperatures overlaid on a side view of Photosystem I (same coloring as in (a)) and an energy-level scheme. Upon excitation of P700 a charge-separated state across the membrane is formed. Interestingly, also the red chlorophyll states (red) with lower excitation energies than P700 are involved in energy transfer. c) The transfer towards P700 is partially blocked and the red chlorophyll states become strongly fluorescent at low tempera- tures. d) The energetic arrangement of the different chromophores allows an effective excitation of the antenna system at the maximum of absorption. The large spectral separation of the maximum of absorption (green) and the fluorescence emission (dark red) ensures an efficient collection of the emission without restrictions by the edge filter (grey) used for blocking of stray light from the excitation source e) A typical Photosystem I fluorescence emission spectrum from an individual Photosystem I complex. The spectra are composed of contributions from different red chlorophyll emitters. Their line widths differ, mainly due to unequal spectral diffusion. The theoretical line shapes of single emitters that are not affected by spectral diffusion are given in red.
