Super-Resolution Microscopy
Being part of the SFB 1101 "Molecular Encoding of Specificity in Plant Processes", we work on establishing super-resolution microscopy (STED, PALM/STORM) in combination with spectroscopy (Nano-FRET) for the detection and analysis of BRI1-containing protein complexes in the plasma membrane.
To circumvent the classical resolution limit (due to the diffractive properties of light) in optical microscopy, several super-resolution principles were established in the last decades - and recently being awarded the Nobel Prize for Chemistry in 2014.
One example method is STED (stimulated emission depletion) microscopy, which can be appended to a CLSM microscope and requires the superposition of two laserfoci. Whereas the excitation laser beam will excite fluorophores in a diffraction-limited spot, a second red-shifted and doughnut-shaped "depletion" laser beam drives affected fluorophores back to the ground state via stimulated emission. As the depletion laser focus exhibits a power dependent intensity minimum in its center, fluorophores in the center remain unaffected and can return to the ground state by emitting fluorescence. As a result, the actual spot from which fluorescence can be detected will be much smaller than the original, diffraction-limited excitation spot.
Another example method is localization microscopy, which is a widefield technique using high-resolution CMOS- or EMCCD-cameras, often operated under TIRF (total internal reflection) illumination. Whether being called PALM (photoactivatable localization microscopy) or STORM (stochastic optical reconstruction microscopy), the underlying principle is the same. Here, the fluorophores are excited in a controlled fashion so that in every image frame only a small fraction of fluorophores is being excited and their diffraction-limited fluorescence spots are well separated. The centers of these spots can be determined mathematically and yield a set of fluorophore coordinates. Each image frame consists of a different set of fluorophores, so by assembling all determined fluorophore coordinates, an image can be reconstructed with much higher fluorophore position accuracy and therefore better resolution. The main difficulty is the controlled on- and off-switching of fluorophore subsets - this can be done e.g. with photoactivatable fluorophores using illumination protocols with different wavelengths or by chemically adjusting the medium of special fluorophores to induce statistical blinking.
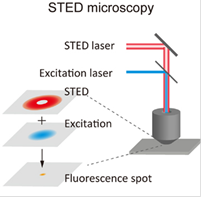
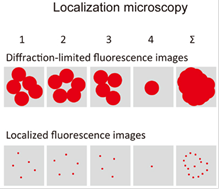
Super-resolution microscopy. Both techniques can theoretically improve the classical optical resolution of ≈200nm by a factor of 10 to ≈20nm. Left: principle of STED. Right: principle of PALM/STORM. Drawings from Habuchi, Front. Bioeng. Biotechnol. 2, 2014
We are currently working to integrate and combine these techniques with our already established methods of spectromicroscopy. While we aim to use STED to resolve fast dynamic cell processes in conjunction with FLIM, the planned highly versatile and combinable TIRF/PALM/STORM setup will prove especially beneficial for separating super-resolution images of plant membranes from their inherent high autofluorescence background.
