Press Releases Archive
16.10.2018
Antilock brake system in arteries protects against heart attack
Researchers at the University of Tübingen have discovered a natural mechanism that blocks the formation of dangerous blood clots
Tübingen biochemists have discovered a natural mechanism of the body that can reduce the formation of dangerous blood clots, also known as thrombosis. So far, this antiblocking system has mainly been studied in mouse arteries. Initial studies with human cells have confirmed the results suggesting that this protective mechanism is highly likely to exist in humans as well. Thrombosis is a leading cause of death worldwide because it can block blood vessels causing heart attack or stroke. The newly discovered mechanism could help to improve therapeutic treatments. The study was conducted by a team of researchers including Dr. Lai Wen and Professor Robert Feil from the Interfaculty Institute of Biochemistry of the University of Tübingen in collaboration with the University Hospital Tübingen and the Universities of Lübeck and Würzburg. Their results have now been published in the journal Nature Communications.
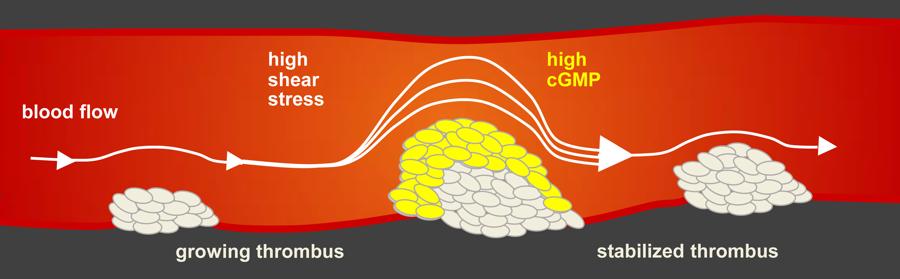
Our body closes wounds when blood platelets glue to the damaged vessel walls and blood clots. This happens externally when we cut our fingers, but also internally at small injuries inside vessels. The latter becomes a problem when the blood clot becomes too large and blocks the blood vessel.
"We have discovered a self-regulating mechanism in platelets of mice and humans that prevents the uncontrolled growth of a blood clot," says Lai Wen, lead author of the study. When a blood clot grows within a vessel, the blood needs to flow around the obstruction. The larger the clot the more force passing blood creates. This “shear stress” acting on the clot increases. This initiates a mechanism that produces more cyclic guanosine monophosphate (cGMP) in the glued platelets. "This messenger molecule prevents further platelets from settling in the area and the life threatening clot slowly dissipates," Wen explains. The bloodcan again flow unrestricted and as the shear stress drops the mechanism stops. A small clot remains and closes the damaged vessel wall. cGMP acts as a kind of antiblocking system for blood vessels, which switches itself on or off as required via shear stress.
"Studies have shown that people who have a genetic defect resulting in less cGMP are at greater risk of heart attacks - the newly discovered mechanism is a possible explanation as to why," adds Feil. The discovery of the cGMP antiblocking system not only gives us a better understanding of how heart attacks develop, but it also opens up new possibilities in the treatment of thrombosis. There are already drugs that support the body in the formation of cGMP. "They were developed for other purposes, but might also be used to treat thrombosis," says Feil. These include, for example, riociguat or sildenafil. The latter is often used in potency-enhancing pills.
"Conventional antithrombotic medicines can cause dangerous bleeding because they affect blood clotting throughout the entire body. On the other hand, drugs that target the cGMP mechanism should not increase the bleeding risk," explains Feil. They are supposed to work only at high shear stress, which is not present outside of blood vessels. However, to confirm this for humans, clinical studies still have to follow. "The relationship between mechanical force acting on cells and the formation of cGMP could also play a role in many other diseases", suspects Feil. "Interesting aspects for future research are the effects of the newly discovered mechanism on blood pressure, osteoporosis or cancer."
Publication:
Lai Wen, Susanne Feil, Markus Wolters, Martin Thunemann, Frank Regler, Kjestine Schmidt, Andreas Friebe, Marcus Olbrich, Harald Langer, Meinrad Gawaz, Cor de Wit, Robert Feil. A shear-dependent NO-cGMP-cGKI cascade in platelets acts as an auto-regulatory brake of thrombosis. Nature Communications. DOI 10.1038/s41467-018-06638-8.
Contact:
Prof. Dr. Robert Feil
University of Tübingen
Interfaculty Institute of Biochemistry (IFIB)
+49-7071-29 73 350
robert.feil@uni-tuebingen.de
Contact for press:
Eberhard Karls Universität Tübingen
Public Relations Department
Dr. Karl Guido Rijkhoek
Director
Antje Karbe
Press Officer
49 7071 29-76789
Fax +49 7071 29-5566
antje.karbe@uni-tuebingen.de
www.uni-tuebingen.de/en/university/news-and-publications.html