Press Releases Archive
15.09.2022
Gut microbes and humans on a joint evolutionary journey
Researchers discover simultaneous evolutionary history of gut microbes with their human hosts over hundreds of thousands of years
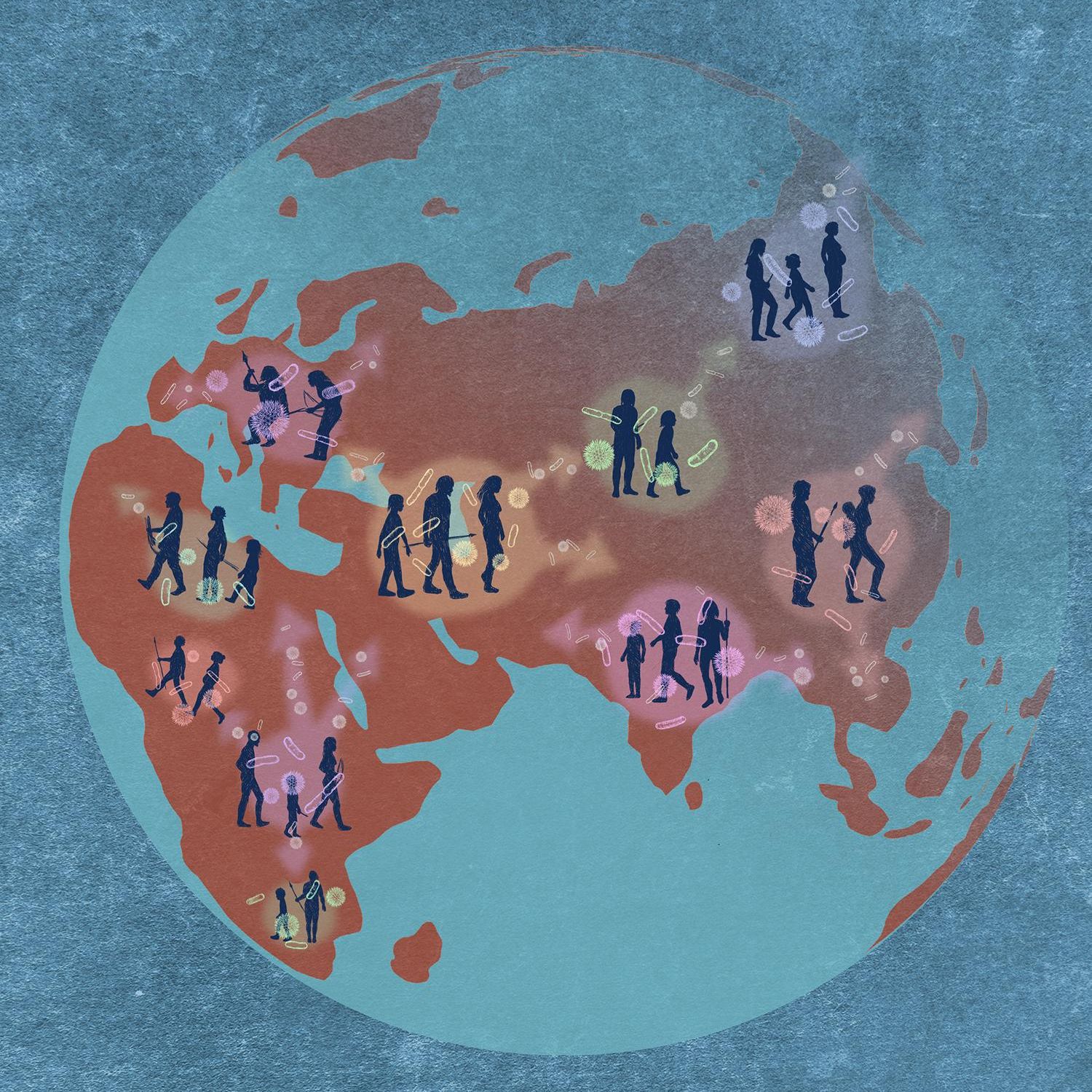
The human gut microbiome is composed of thousands of different bacteria and archaea that vary widely between populations and individuals. Scientists from the Max Planck Institute for Biology in Tübingen have now discovered gut microbes that share a parallel evolutionary history with their human hosts: the microorganisms co-evolved in the human gut environment over hundreds of thousands of years. In addition, some microbes exhibit genomic and functional features making them dependent on their host. Now published in Science, the researchers present the results of their study conducted with data from 1225 individuals out of Africa, Asia and Europe.
Many microbe species in the human gut can be found across populations from all over the world. However, within a microbe species the microbe strains vary remarkably between individuals and populations. Despite their importance for human health, little was known so far about the origins of these strains. Moreover, most of these strains live almost exclusively in the human gut. This raises the question of where the microorganisms in the human gut come from.
Linking the histories of microbes and humans
The research team conjectured that specific species and strains have been with people as humanity diversified and spread over the globe. To test if microbes evolved and diversified simultaneously with their human hosts, researchers from the Max Planck Institute for Biology, the Institute for Tropical Medicine, and the Cluster of Excellence CMFI at the University of Tübingen systematically compared for the first time the evolutionary histories of humans and of gut microbes. The researchers created phylogenetic trees for 1225 human study participants as well as for 59 microbial species found within their guts, and used statistical tests to investigate how well these trees match. Over 60% of the investigated species matched with the evolutionary history of their human host, meaning that these microbes co-diversified over ~100,000 years in the human gut when people fanned out of Africa across the continents. “We didn`t know that any of our gut microbes followed our evolutionary history this closely”, marvels Ruth Ley, head of the department for Microbiome Science at the Max Planck Institute for Biology, Tübingen, where the study was conducted, and deputy spokesperson of the CMFI.
Gut microbes became dependent on their hosts
“It is also remarkable that the strains that followed our history most closely are now those who rely most on the gut environment,” Ley adds. Indeed, some of the microbe strains that evolved together with humans are heavily dependent on the human gut environment: they possess smaller genomes and are more sensitive to oxygen levels and temperature – traits making it difficult to survive outside the human body. In contrast, microorganisms that showed weaker association with the human history showed more characteristics similar to free living bacteria. “Some of the gut microbes behave like they are part of the human genome”, explains Taichi Suzuki, who shares main authorship of the study with his colleague Liam Fitzstevens. Suzuki adds: “You can imagine that those microbes are on a gradient from ‘free-living’ to reliant on the human body environment. We have seen that some human gut bacteria are further along the gradient towards irreversible host dependence than previously thought.” Ley further states: “This fundamentally changes how we view the human gut microbiome.”
Population-specific approach to microbiome-based therapies
To obtain data from a diverse subset of the global population, the research team analyzed the gut microbes and genomes of 1225 individuals in Europe, Asia, and Africa. The stool and saliva samples were collected with the help of researchers from the Institute for Tropical Medicine at the University of Tübingen and their partners in Vietnam and Gabon. In addition, researchers around the globe supported the study by providing similar datasets from participants recruited in Cameroon, South Korea, and the UK.
The findings of the study help to further understand population-specific microbes that have long been associated with the local human population. With this knowledge, microbiome-based therapies of diseases can be adapted and refined to a population-specific treatment.
Original publication:
Taichi A. Suzuki, J. Liam Fitzstevens, Victor T. Schmidt, Hagay Enav, Kelsey E. Huus, Mirabeau Mbong Ngwese, Anne Grießhammer, Anne Pfleiderer, Bayode R. Adegbite, Jeannot F. Zinsou, Meral Esen, Thirumalaisamy P. Velavan, Ayola A. Adegnika, Le Huu Song, Timothy D. Spector, Amanda L. Muehlbauer, Nina Marchi, Hyena Kang, Lisa Maier, Ran Blekhman, Laure Ségurel, GwangPyo Ko, Nicholas D. Youngblut, Peter Kremsner, Ruth E. Ley: Codiversification of gut microbiota with humans. Science vol. 377, issue 6612 (2022).
Press release by the Max Planck Institute of Biology Tübingen
Scientific contact:
Taichi A. Suzuki, Ph.D.
Max Planck Institute for Biology Tübingen
Department of Microbiome Science
Max-Planck-Ring 5
72076 Tübingen, Gemany
Email: taichi.suzuki@tuebingen.mpg.de
Phone: +49 7071 601 1321
Website: taichisuzuki.weebly.com
Liam Fitzstevens
Max Planck Institute for Biology Tübingen
Department of Microbiome Science
Max-Planck-Ring 5
72076 Tübingen, Germany
Email: liam.fitzstevens@tuebingen.mpg.de
Phone: +49 7071 601 401
Prof. Ruth E. Ley, Ph.D.
Max Planck Institute for Biology Tübingen
Director, Department of Microbiome Science
Max-Planck-Ring 5
72076 Tübingen, Germany
Email: rley@tuebingen.mpg.de
Phone: +49 7071 601 450
Website: https://leylab.com/